X-Ray Fluorescence (XRF)
PXRF and XRF Technology
Established in 2009, Portable Analytical Solutions (PAS) delivers comprehensive knowledge and support across the range of Portable (PXRF) and Non Portable XRF Equipment.
We have the perfect solution no matter what industry or application you are after. Take a look at our XRF products.
Applications & Industries for XRF & PXRF
Our Range Of Portable & Non-Portable X-Ray Fluorescence Equipment Includes:
- Niton XL2 (Portable Handheld XRF)
- Niton XL2 Plus (Portable Handheld XRF)
- Niton XL3 (Portable Handheld XRF)
- Niton XL5 (Portable Handheld XRF)
- Niton DXL (Bench Top XRF)
What is XRF?
X-ray Fluorescence (XRF) is a non-destructive analytical technique to determine the elemental composition of materials.
XRF analysers determine the chemistry of a sample by measuring the fluorescent (or secondary) X-ray emitted from a sample when it is excited by a primary X-ray source.
Each element present in a sample produces a unique set of characteristic fluorescent X-rays (“a fingerprint”) which is why XRF spectroscopy is ideal for qualitative and quantitative analysis of material composition.
An XRF analyser uses the X-Ray Fluorescence process of irradiating a solid or liquid sample and measuring differences in the various quantum states of the sample atom’s electrons.
PAS provides leading solutions for XRF analysis and accessories.
With advancements in technology and solutions, Handheld XRF or Portable XRF (PXRF) units are now becoming more integral to real time, in field analysis. Innovation in the field has allowed further advancements to the way the operators leverage these analysers to source data and information faster than ever before.



The X-Ray Fluorescence Process
- A solid or a liquid sample is irradiated with high energy X-rays from a controlled X-ray tube.
- When an atom in the sample is struck with an X-ray of sufficient energy (greater than the atom’s K or L shell binding energy), an electron from one of the atom’s inner orbital shells is dislodged.
- The atom regains stability, filling the vacancy left in the inner orbital shell with an electron from one of the atom’s higher energy orbital shells.
- The electron drops to the lower energy state by releasing a fluorescent X-ray. The energy of this X-ray is equal to the specific difference in energy between two quantum states of the electron. The measurement of this energy is the basis of XRF analysis.
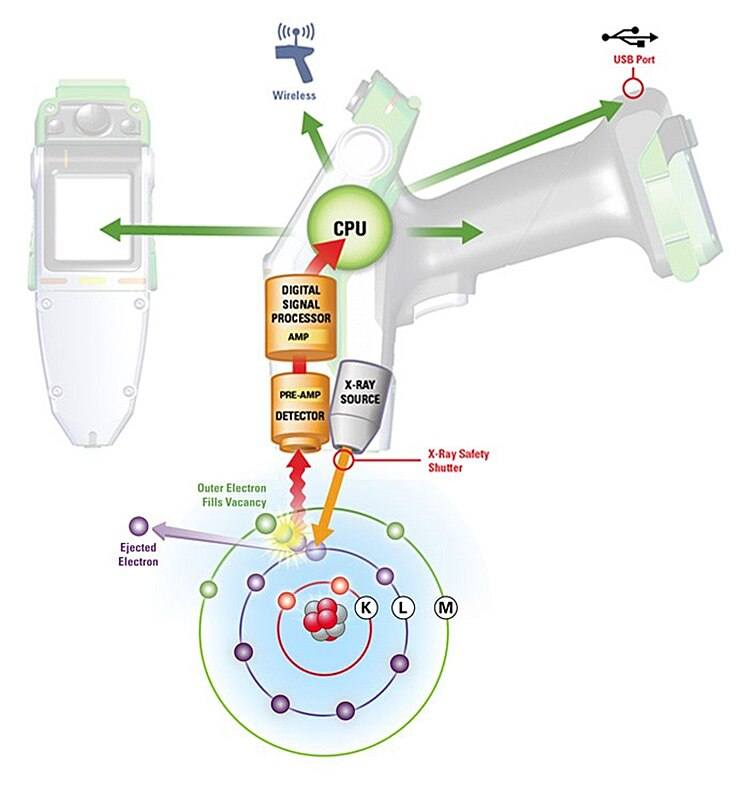
Interpretation of XRF Spectra
Most atoms have several electron orbitals (K shell, L shell, M shell, for example). When x-ray energy causes electrons to transfer in and out of these shell levels, XRF peaks with varying intensities are created and will be present in the spectrum, a graphical representation of X-ray intensity peaks as a function of energy peaks. The peak energy identifies the element, and the peak height/intensity is generally indicative of its concentration.
Energy Dispersive X-Ray Fluorescence (EDXRF)
EDXRF is the technology commonly used in portable x-ray fluorescence analyzers.
EDXRF is designed to analyze groups of elements simultaneously in order to rapidly determine those elements present in the sample and their relative concentrations—in other words, the elemental chemistry of the sample.
To understand how this information can be used, consider scrap metal. Recyclers need to positively identify numerous alloy grades, rapidly analyze their chemical composition at material transfer points, and guarantee the quality of their product to their customers. Metal alloys are designed for specific functions that are not interchangeable; small variations in composition can result in significantly different mechanical properties. Luckily, handheld XRF analyzers can easily separate these grades.
To learn more about XRF, you can read our article where we explain what XRF is.
Which XRF Instrument Do You Need?
Whilst all of our XRF & PXRF units undertake a similar process for analysing materials and metals, they each serve a unique purpose.
Niton XL2 PXRF
This analyser provides immediate, nondestructive elemental analysis for a wide range of applications. It can detect alloy materials from titanium to nickel, as well as tramp and trace element analysis. The XL2’s standard analytical range spans up to 30 elements from sulphur to uranium.
Niton XL3 PXRF
This portable XRF unit is designed for specific industry applications. A tilting, colour, touch-screen display allows easy viewing of sample results under any condition. The optional integrated camera allows users to locate, view, and store the analysis image and the test results for later reference.
Niton DXL Portable Benchtop XRF
This analyser is typically used by jewellers and pawn shops. The Niton gold spectrometers quickly provide the exact karat weight and percentages of all elements within an item – easily identifying non-standard, under-karated, and even advanced counterfeit gold with fire assay-comparable accuracy.
Niton XL2 Plus PXRF
This is a tough, powerful, handheld instrument for identifying metal alloys and mineral ores easily in the field. It can identify Niobium in Titanium alloys down to 200 ppm and more generally, elements from Magnesium to Uranium, down to 20 ppm in common alloys.
Niton XL5 PXRF
This top range model has the best limits of detection in the range, whilst being smaller and lighter. The XL5 allows the x-ray source and fluorescence detector to be closer to the sample, improving limits of detection and shortening measurement time, especially for light elements. As well as metals, the XL5 measures the elemental composition of scale, sludge, oil, powders and slurries.
Sample Preparation Benchtop XRF
Available in 3 different models, this analyser uses electrical fusion technology to prepare samples before using XRF to determine the material.
For more information, you can read our guide to PXRF instruments.
Contact Us To Discuss XRF's Today
If you’d like to discuss XRF’s and PXRF’s in further detail, you can reach us by phone or by filling out our contact form




